Mark Hines
asked on
Fuel Volume Formula
What do I multiply my gallons of jet fuel to get true volume based on temperature.??
I think somewhere it was discussed but I can not find it now.
I think somewhere it was discussed but I can not find it now.
You need to know the density of the fuel as a function of temperature.
ASKER
.82 (Jet A)
Can I email you directly..???
Can I email you directly..???
ASKER
Somewhere you wrote what factor to multiply the gallons by for very degree over 60 Deg F.
but I can't seem to find it now.
but I can't seem to find it now.
ASKER
I have a 10,000 gallon tank that I am measuring the height and calculating the volume.
But I read somewhere that temperature has to be taken into effect.
For every degree over 60 you multiply the gallons by this factor to get the compensated value.
But I read somewhere that temperature has to be taken into effect.
For every degree over 60 you multiply the gallons by this factor to get the compensated value.
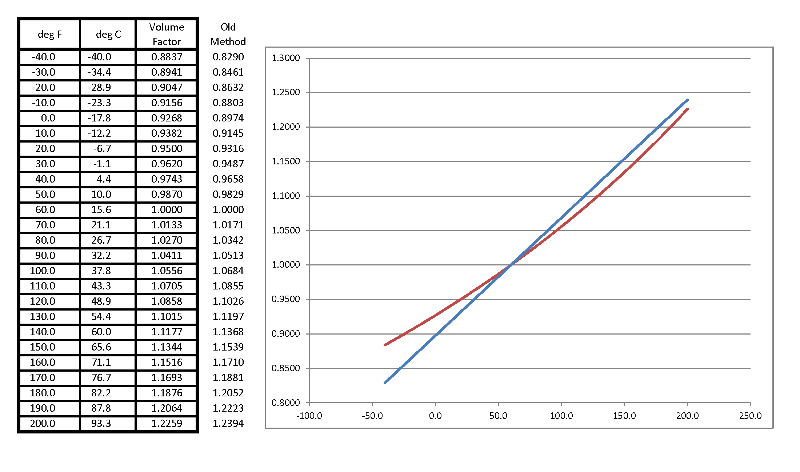
I think the data you need are on pages 20 (formula) and 22 (graph) of this document:
http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&cad=rja&uact=8&ved=0CCcQFjAB&url=http%3A%2F%2Fdtic.mil%2Fdtic%2Ftr%2Ffulltext%2Fu2%2Fa132106.pdf&ei=0gUzVJmsFq2asQSTvYG4DA&usg=AFQjCNFV8HPrqky_rRaw9RKLrwZahQgX3g&sig2=NpsB0N9K7O84TzNOqdnOPg&bvm=bv.76802529,d.cWc
http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&cad=rja&uact=8&ved=0CCcQFjAB&url=http%3A%2F%2Fdtic.mil%2Fdtic%2Ftr%2Ffulltext%2Fu2%2Fa132106.pdf&ei=0gUzVJmsFq2asQSTvYG4DA&usg=AFQjCNFV8HPrqky_rRaw9RKLrwZahQgX3g&sig2=NpsB0N9K7O84TzNOqdnOPg&bvm=bv.76802529,d.cWc
The formula on page 20 is not relevant, but here is the data:
You can measure either volume or mass.
The volume will change with temperature, the mass won't.
So if you have a tank with a measured volume and a known temperature, you can calculate the mass or the volume at the standard temperature which appears to be 15 deg C (60 deg F).
Density is linear with temperature, but volume will not be. How accurate do you need to be?

You can measure either volume or mass.
The volume will change with temperature, the mass won't.
So if you have a tank with a measured volume and a known temperature, you can calculate the mass or the volume at the standard temperature which appears to be 15 deg C (60 deg F).
Density is linear with temperature, but volume will not be. How accurate do you need to be?
ASKER
Im trying to calculate my net and gross gallons..
In an earlier post you said....
For every degree above 60F, multiply by 0.00171, subtract from 1.0, that's your conversion factor from hot gallons to standard gallons. For example a factor of 0.900 means one hot gallon is actually 0.9 standard gallons.
And for every degree below 60F, multiply by 0.00171, add to 1.0, that's your conversion factor from cold gallons to standard gallons.
What do you mean by subtract from 1.0 and add to 1.0..????
How does that work into the formula..???
In an earlier post you said....
For every degree above 60F, multiply by 0.00171, subtract from 1.0, that's your conversion factor from hot gallons to standard gallons. For example a factor of 0.900 means one hot gallon is actually 0.9 standard gallons.
And for every degree below 60F, multiply by 0.00171, add to 1.0, that's your conversion factor from cold gallons to standard gallons.
What do you mean by subtract from 1.0 and add to 1.0..????
How does that work into the formula..???
Try
Gallons(T) =. Gallons(60) * ( 1 + (T-60)*Factor)
Gallons(T) =. Gallons(60) * ( 1 + (T-60)*Factor)
I don't remember the earlier post, but I will get back to this tomorrow
What units are you using?
ASKER
Volume = US Gallons
Temperature = Degrees F
Max Tank Size = 50,000 Gallons
Temperature = Degrees F
Max Tank Size = 50,000 Gallons
ASKER
FYI...
I measure the fuel height in Inches to calculate the volume in Gallons.
Im using this all in a PLC (AB Micrologix 1500).
Im trying to calculate the Gross Gallons and Net Gallons to display on my HMI.
Im already displaying the height (inches), temperature (degrees F).
I measure the fuel height in Inches to calculate the volume in Gallons.
Im using this all in a PLC (AB Micrologix 1500).
Im trying to calculate the Gross Gallons and Net Gallons to display on my HMI.
Im already displaying the height (inches), temperature (degrees F).
ASKER
I apologize if Im making this harder than it is, but I need to make sure I understand exactly what I need to do.
This is what I think you are telling me, but please correct me if I am wrong.
Gross Gallons = Height measured converted to Volume (Gallons).
Net Gallons = Temperature Compensated Volume (Gallons).
Net Gallons = (Gross Gallons) x ((Temp - 60) + 1) x .00171
Please show revised formula if this is not correct.
Thank you in advance
This is what I think you are telling me, but please correct me if I am wrong.
Gross Gallons = Height measured converted to Volume (Gallons).
Net Gallons = Temperature Compensated Volume (Gallons).
Net Gallons = (Gross Gallons) x ((Temp - 60) + 1) x .00171
Please show revised formula if this is not correct.
Thank you in advance
The final result would be:
771.1
Net Gallons = Std Gallons ( ------------------ )
832 - 1.015*TempASKER
The last formula is hard to read because it did not seem to displayu correctly..sorry.
So IF I had 25000 gallons at 80 degrees F ...
Volume Corrected = 25000 x 771.1/832 - (1.015 x 80)
Is this correct..???
So IF I had 25000 gallons at 80 degrees F ...
Volume Corrected = 25000 x 771.1/832 - (1.015 x 80)
Is this correct..???
ASKER
X = Volume (Gallons) Value that is Temperature Corrected (25,675.94)
Y = Degrees F of Fuel (80)
Z = Volume (Gallons) Value that is "NOT" Temperature Corrected (25,000)
X = (Z x 771.1) divided by 832 minus (1.015 x Y)
X = (25,000 x 771.1) divided by 832 minus (1.015 x 80)
X = (19,277,500) divided by 832 minus (81.2)
X = 19,277,500 divided by 750.8
X = 25,675.94
Please let me know if this is correct or not if you would.
IF this is not what you met, please correct me.
Thanks for all your help.
Y = Degrees F of Fuel (80)
Z = Volume (Gallons) Value that is "NOT" Temperature Corrected (25,000)
X = (Z x 771.1) divided by 832 minus (1.015 x Y)
X = (25,000 x 771.1) divided by 832 minus (1.015 x 80)
X = (19,277,500) divided by 832 minus (81.2)
X = 19,277,500 divided by 750.8
X = 25,675.94
Please let me know if this is correct or not if you would.
IF this is not what you met, please correct me.
Thanks for all your help.
ASKER
Well...I can now see that I am interpreting your answer incorrectly,
so if you would please let me know what I am doing wrong.
so if you would please let me know what I am doing wrong.
>> So IF I had 25000 gallons at 80 degrees F ...
The terms Net, Standard, Corrected, Gross, and Actual are all kind of confusing. 60 deg F seems to be the standard temperature, but I am going to use the number not the word.
You have is a volume of fuel at 80 deg F, and you want to know what the volume would be at 60 deg F.
The formula tells you what happens to a volume of fuel that starts at 60 deg F and warms up or cools down. The final temperature here is 80 deg F.
V_80 = V_60 * [(771.10) / (832 - 1.015*80)]
Calculate the factor in the square brackets first. You should get a number near one: 1.0270 in this case. You can check it on the Excel chart or plot.
The final expression is: V_80 = V_60 * 1.0270
And the answer: V_60 = 25000 / 1.0271 = 24343 gallons
The terms Net, Standard, Corrected, Gross, and Actual are all kind of confusing. 60 deg F seems to be the standard temperature, but I am going to use the number not the word.
You have is a volume of fuel at 80 deg F, and you want to know what the volume would be at 60 deg F.
The formula tells you what happens to a volume of fuel that starts at 60 deg F and warms up or cools down. The final temperature here is 80 deg F.
V_80 = V_60 * [(771.10) / (832 - 1.015*80)]
Calculate the factor in the square brackets first. You should get a number near one: 1.0270 in this case. You can check it on the Excel chart or plot.
The final expression is: V_80 = V_60 * 1.0270
And the answer: V_60 = 25000 / 1.0271 = 24343 gallons
ASKER
Hi Again..
You say above that the density at 60 Deg F is 771.1
but everything I have says that the density of Jet A at 60 Deg F is .82
Am I missing something..???
You say above that the density at 60 Deg F is 771.1
but everything I have says that the density of Jet A at 60 Deg F is .82
Am I missing something..???
Sorry for the delay and the confusion.
My calculations have been bad, but my physics has been worse.
I am going to start clean, rather than try to find the earlier error.
>> You say above that the density at 60 Deg F is 771.1 but everything I have says
that the density of Jet A at 60 Deg F is .82
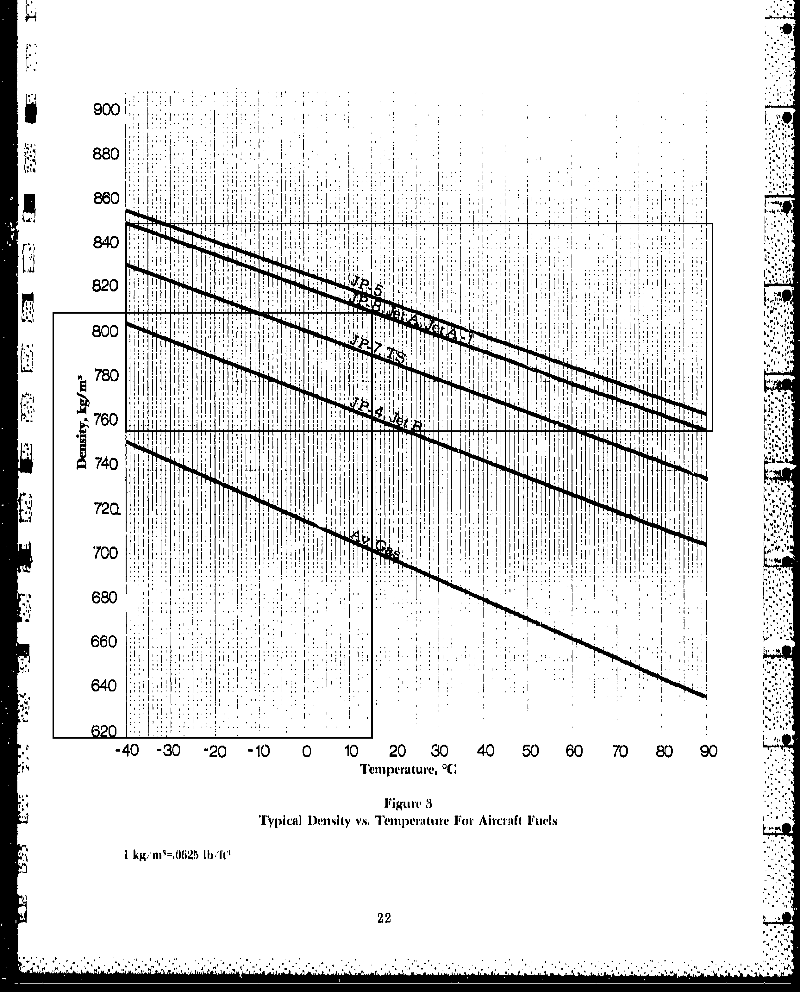
The Typical Fuel Density Chart (which is the basis for this analysis) indicates a density
for Jet_A at 15 deg C ( = 59 deg F) slightly less than 0.81
 These six points all check out.
These six points all check out.
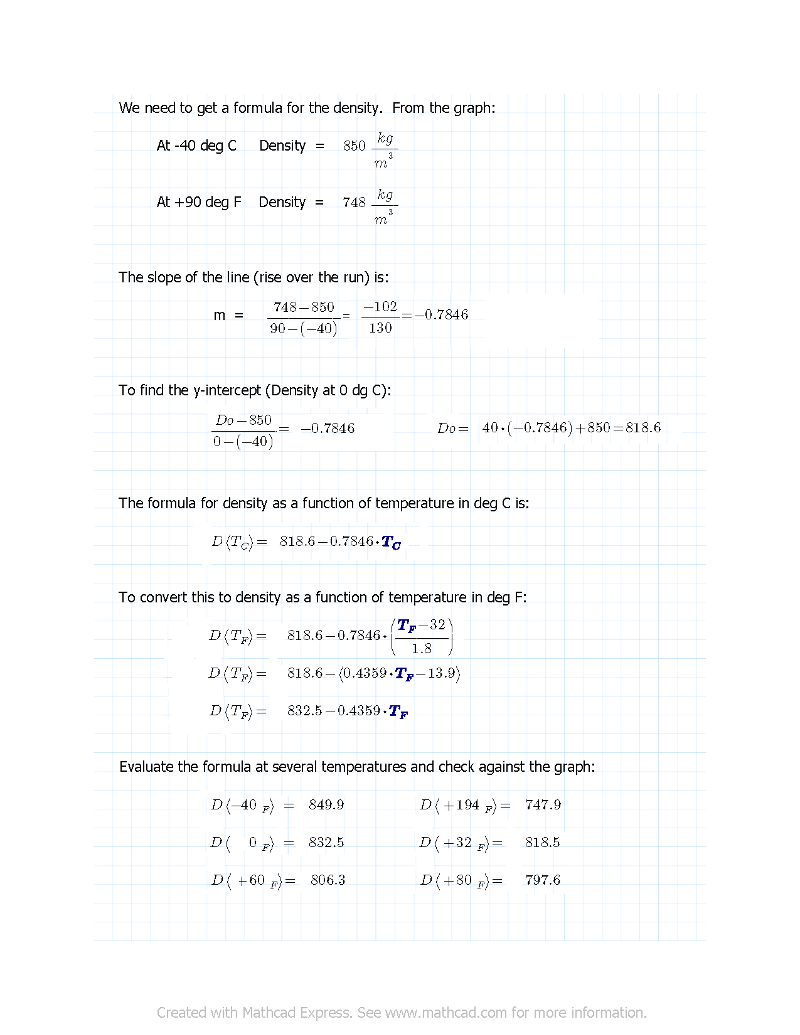
The equation you need is:
V_60*D_60 = V_T*D_T
V_60 = V_T * (832.5 - 0.4359*T_F) / (806.3)
For 25,000 gallons at 80 deg F:
V_60 = 0.9892 * 25,000 = 24,730
V_60 = V_80*(D_80/D_60) = V_80*(797.6/806.3) = 0.9892*V_80
V_60 = 0.9892 * 25,000 = 24,730
My calculations have been bad, but my physics has been worse.
I am going to start clean, rather than try to find the earlier error.
>> You say above that the density at 60 Deg F is 771.1 but everything I have says
that the density of Jet A at 60 Deg F is .82
The Typical Fuel Density Chart (which is the basis for this analysis) indicates a density
for Jet_A at 15 deg C ( = 59 deg F) slightly less than 0.81
 These six points all check out.
These six points all check out.The equation you need is:
V_60*D_60 = V_T*D_T
V_60 = V_T * (832.5 - 0.4359*T_F) / (806.3)
For 25,000 gallons at 80 deg F:
V_60 = 0.9892 * 25,000 = 24,730
V_60 = V_80*(D_80/D_60) = V_80*(797.6/806.3) = 0.9892*V_80
V_60 = 0.9892 * 25,000 = 24,730
ASKER
Can you make this simpler to understand..??
Just a simple formula that I can substitute the different Temps and Gallons..!!
Im limited in my controller what I can do.
Thanks
Just a simple formula that I can substitute the different Temps and Gallons..!!
Im limited in my controller what I can do.
Thanks
ASKER
Example:
X = Temperarure
Y = Measured Gallons
What formula can I use that when the X and Y varies it will
give me the true "Temperature Compensated" gallons..??
X = Temperarure
Y = Measured Gallons
What formula can I use that when the X and Y varies it will
give me the true "Temperature Compensated" gallons..??
This would be the PLC formula: V_60 = V_T * (832.5 - 0.4359*T_F) / (806.3)
V_T is the volume of fuel in gallons at some temperature T_F in deg F.
(832.5 - 0.4359*T_F) is the density of the fuel at T_F.
(806.3) is the density of the fuel at 60 deg F.
V_60 is the value you want, the volume of your fuel at 60 deg F.
V_T is the volume of fuel in gallons at some temperature T_F in deg F.
(832.5 - 0.4359*T_F) is the density of the fuel at T_F.
(806.3) is the density of the fuel at 60 deg F.
V_60 is the value you want, the volume of your fuel at 60 deg F.
As a check, this is what the formula gives at a few temperatures:
20.00 102.17
30.00 101.63
40.00 101.09
50.00 100.55
60.00 100.01 <== Small round-off error
70.00 99.47
80.00 98.92
90.00 98.38
100.00 97.84ASKER
All the FAA guys here say I must use a value of .82 for the density value of Jet A fuel.
That value of 806.3 is confusing us..
That value of 806.3 is confusing us..
ASKER CERTIFIED SOLUTION
membership
This solution is only available to members.
To access this solution, you must be a member of Experts Exchange.
ASKER
This final answer is not even close to being correct..!!!!